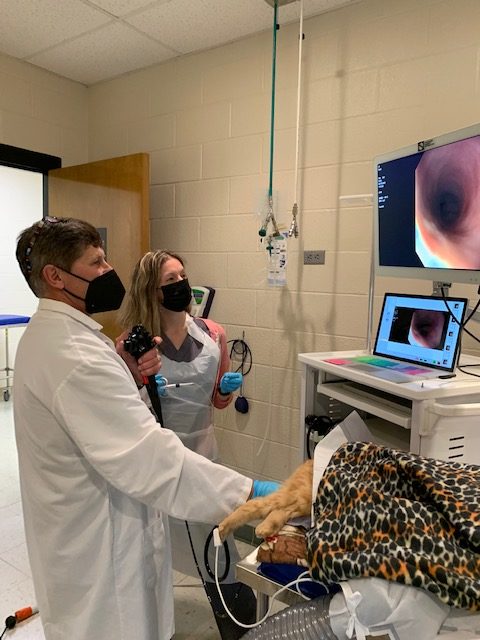
By Dr. Tracy Webb and Dr. Kristen Weishaar
On May 20, Clinical Trials Day is celebrated around the world to recognize the people who conduct clinical research, and to raise awareness about how clinical trials benefit all living things. The event acknowledges the day – May 20, 1747 – the first randomized clinical trial was started by James Lind, aboard a ship, to determine the best treatment for scurvy.
At Colorado State University, researchers conduct hundreds of clinical trials that yield important insights for human and animal health.
What are clinical trials?
Clinical trials are research studies that involve human or veterinary clinical patients, after obtaining informed consent. Clinical trials take information learned in the laboratory into studies with actual patients to help determine if they are safe and effective. Trials are used in all specialties of medicine to evaluate new ways to detect and treat diseases. Many of the standard treatments used today are the result of clinical trials. We learn valuable information from every patient, and we use this information to improve their care as well as the care of future patients, both pets and people.
What are some benefits of clinical trials enrollment?
Clinical trials are designed to help us improve patient outcomes and can have benefits for participants including:
- Exposure to new tests or treatments that may have benefits similar or superior to current standard of care
- Financial incentives
- Treatments and/or diagnostics associated with the study are often paid for by the trial
- May have additional financial compensation at conclusion of study
- Contribution to research with potential benefit in animals and/or humans in the future
What are some potential risks of enrolling in a clinical trial?
Clinical trials can also have some risks that potential participants need to weigh carefully prior to enrolling themselves or their family members, including their pets, in a clinical trial:
- Treatment may not have any effect against disease
- Unknown side effects that could be severe (including death)
- More complete and more invasive diagnostics may be required for eligibility assessment
- Additional visits and/or diagnostics may be required (blood samples, biopsies, etc.) as part of trial participation
All of the study information is discussed during the consenting process and is included in the consent form to ensure participants are informed of all the benefits, risks, and requirements before they agree to enroll in a clinical trial. If you are considering participating in a clinical trial, you should be sure to ask any and all questions prior to enrolling and along the way as the better a participant understands the study, the better the study outcomes will be.
Clinical trials at the CSU Veterinary Teaching Hospital

The James L. Voss Veterinary Teaching Hospital (VTH) is committed to providing the best care for animals, which includes conducting research to find ways to improve detection, diagnosis, and treatment of pet and human illnesses, all while enhancing the quality of care each patient receives. Currently, there are over 150 clinical trials open for enrollment at hospital, involving many different species and diseases. You can learn more about these trials by visiting the hospital’s clinical trials website.
To find out about veterinary clinical trials available at other locations in the US and Canada, the American Veterinary Medical Association’s website hosts a searchable clinical trials database. Information can also be found on individual laboratory and institution’s websites and some specific sites such as the Food and Drug Administration’s website for clinical field studies for animal cells, tissues, and cell- and tissue-based products.
We all benefit from quality clinical trials, and today we thank all those who make them possible.
Dr. Tracy Webb is a veterinarian and research scientist in the Department of Clinical Sciences. Dr. Kristen Weishaar is a veterinary oncologist and director of clinical trials at the Flint Animal Cancer Center.

![Annabelle CTD One Cure2[6]](https://cvmbs.source.colostate.edu/wp-content/uploads/sites/7/2022/05/Annabelle-CTD-One-Cure26-rotated.jpg)
![Cooper1[3]](https://cvmbs.source.colostate.edu/wp-content/uploads/sites/7/2022/05/Cooper13.jpg)